| Overview |
| Objectives |
| Artificial Organic Networks |
| Artificial Hydrocarbon Networks |
| Fundamentals on Organic Chemistry |
Observations to chemical organic compounds provide enough information to inspire the following technique named Artificial Organic Networks.
In organic chemistry, the study of compounds made of carbon elements, it is well known that organic compounds are the most stable compounds. In fact, this stability is due to the energy minimization and the geometry of chemical structures. Another interesting property is that organic compounds are made of a few elements: carbon, hydrogen, oxygen, nitrogen, sulfur and halogens (fluorine, chlorine, bromine, iodine and astatine); and with this finite set of elements there are around 20 million different known compounds. In other words, few elements interacting among them have the power to produce a huge amount of different compounds.
Interestingly, relationships of elements make possible a unit called molecule (two or more atoms interacting among them). Different atoms and different arrangement of those make different molecules. Actually, the differentiation of molecules may be observable from their physical and chemical properties. Translating, physical properties refer to the structure of the molecule while chemical features refer to the behavior of the molecule. Thus, molecules can be seen as basic units of information characterized by the atoms in the inside. Moreover, chemical bonds and chemical balance reactions perform relationships between molecules forming: complex molecules, or a mixture of molecules. The latter is so-called a compound.
In a nutshell, the above observations can be summarized:
- The basic unit with information in organic compounds is the molecule.
- Molecules are characterized by the atoms in the inside.
- Molecules have physical properties (structure) and chemical properties (behavior).
- Chemical bonds relate molecules to perform complex molecules.
- Chemical balance interaction performs compounds (mixture of molecules).
- Easiness of spanning compound structures (creation and addition of molecules).
- Organic compounds are stable via energy minimization.
- A finite set of atoms have the power to produce a huge amount of organic compounds.
Then, Artificial Organic Networks (AONs for short) are inspired on the observations depicted so far. In fact, this technique improves computational algorithms for modeling problems in terms of stability and partial knowledge of model behavior. In order to understand the essence of AONs, the following sections present the components and their interactions, the model of AONs, and the open problems related to this new artificial intelligence technique.
Components
- Atom: Specialized parameter of a molecule.
- Molecule: The basic unit of information with structure (graph) and behavior (mathematical function).
- Complex molecule: Nonlinear mixture of information due to the interaction of molecules via nonpolar covalent bonds.
- Compound: Linear mixture of molecules via chemical balance interaction.
- Stoichiometric coefficients: Weights of the mixture of molecules.
Interactions
- Polar covalent bonds: Relationship between two different atoms.
- Nonpolar covalent bonds: Relationship between two similar atoms based on the chemical model of energy in covalent bonds (refer to the Fundamentals on Organic Chemistry section).
- Chemical balance interaction: Linear combination of different molecules in a certain proportion (stoichiometric coefficients).
Model of Artificial Organic Networks
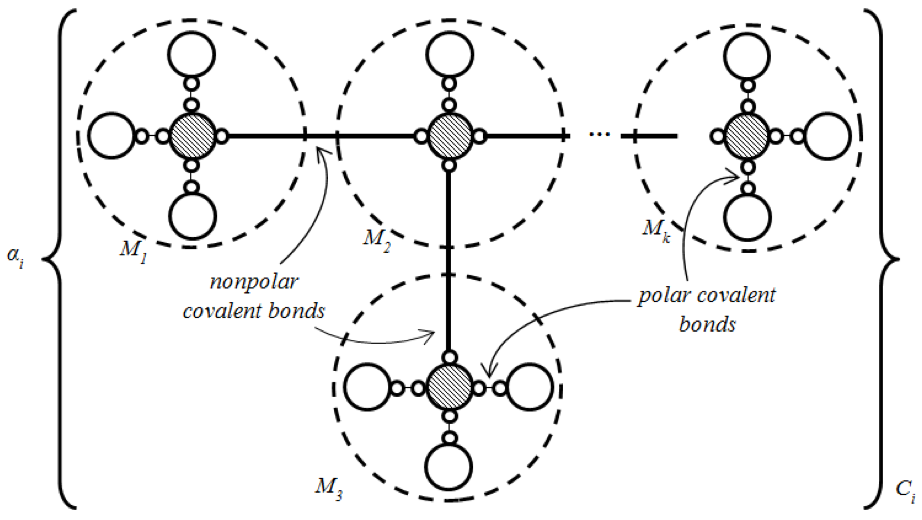
An Artificial Organic Network is a structure of mixture of molecules (compound) defined by the arrangement of the related atoms in order to capture partial knowledge of information due to some input signals of a given unknown system, and to get stability in the algorithm. Figure 1 shows a basic Artificial Organic Network. In fact, both stability and setting atoms in the structure are obtained via energy stabilization, the central observation in chemical organic compounds.
Figure 1. Basic structure of Artificial Organic Networks.In that sense, three levels of energy stabilization are identified in AONs:
- Ground-state allows energy stabilization in atoms.
- Nonpolar covalent bonds minimize energy when relating two highly energized molecules.
- Chemical balance interaction equilibrates energy in molecules.
Figure 2 shows the energy model of Artificial Organic Networks. In addition, a mathematical reference and components of AONs related to each level of energy are also depicted.
<Image not available>Figure 2. Model of energy levels in Artificial Organic Networks.
Open Problems in Artificial Organic Networks
Since AONs are primary computational algorithms for modeling problems, two nontrivial problems are related to them:
- Finding all atom-parameters for a given structure of AONs in order to approximate any given system.
- Finding the structure of AONs (all atoms and bonds) in order to get a stable compound for approximating any given system.
In fact, Artificial Hydrocarbon Networks is a practical approach of AONs based on chemical hydrocarbons.